Clsi E Coli Breakpoints 2024 Schedule
Clsi E Coli Breakpoints 2024 Schedule. Effective january 2024, clinical laboratories performing antimicrobial susceptibility testing (ast) will be required to use breakpoints currently recognized by clinical and laboratory standards. When a cast system is validated and cleared at the fda level, performance characteristics are established using current breakpoints at the time, which are likely tightly.
Aeruginosa led clsi to an update of the aminoglycoside. Quickly reference the most trusted ast veterinary breakpoint tables as a convenient, complimentary supplement to the ast vet01 document.
Clsi E Coli Breakpoints 2024 Schedule Images References :
 Source: carylbmarlane.pages.dev
Source: carylbmarlane.pages.dev
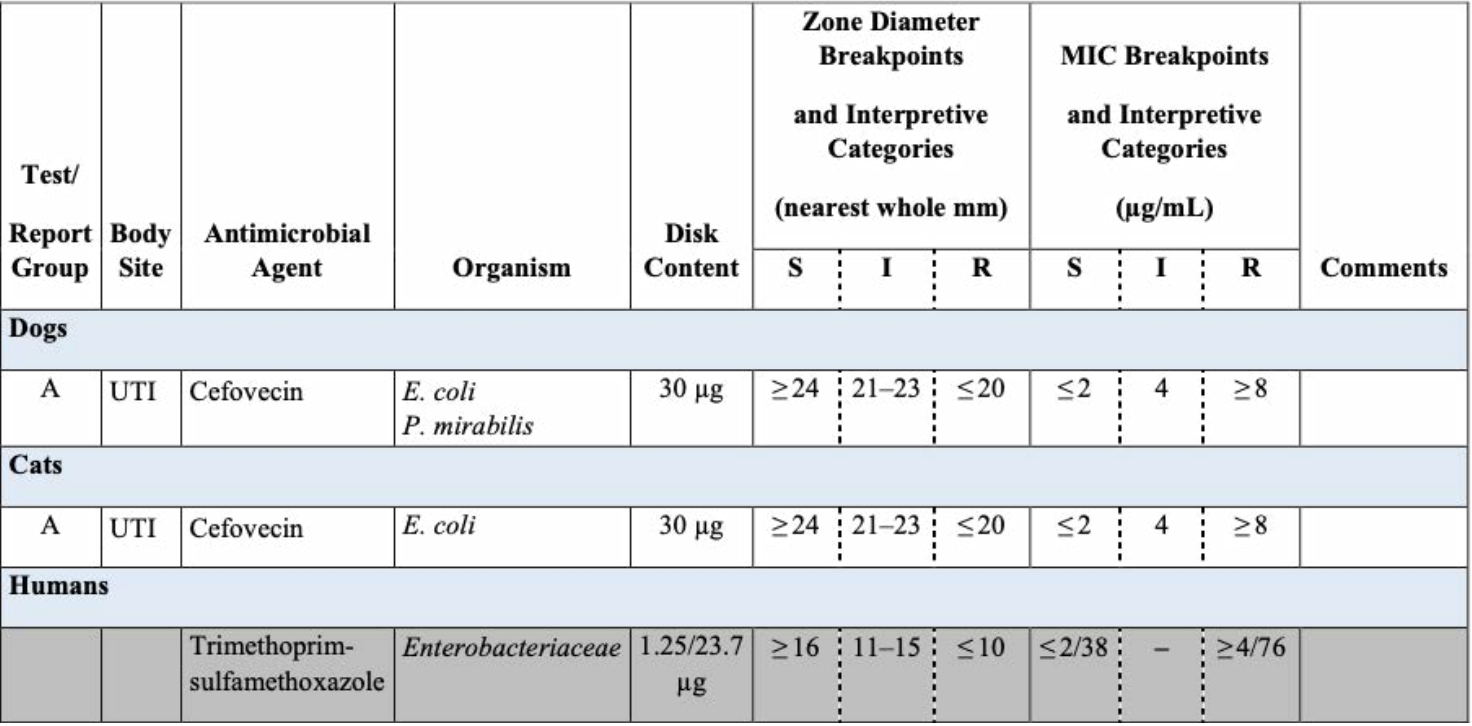
Clsi Breakpoints 2024 E Coli Suzy Leland, Summarize highlights from clsi m100—performance standards for antimicrobial susceptibility testing, 34th edition standards for antimicrobial susceptibility testing (ast) and reporting.
 Source: carylbmarlane.pages.dev
Source: carylbmarlane.pages.dev
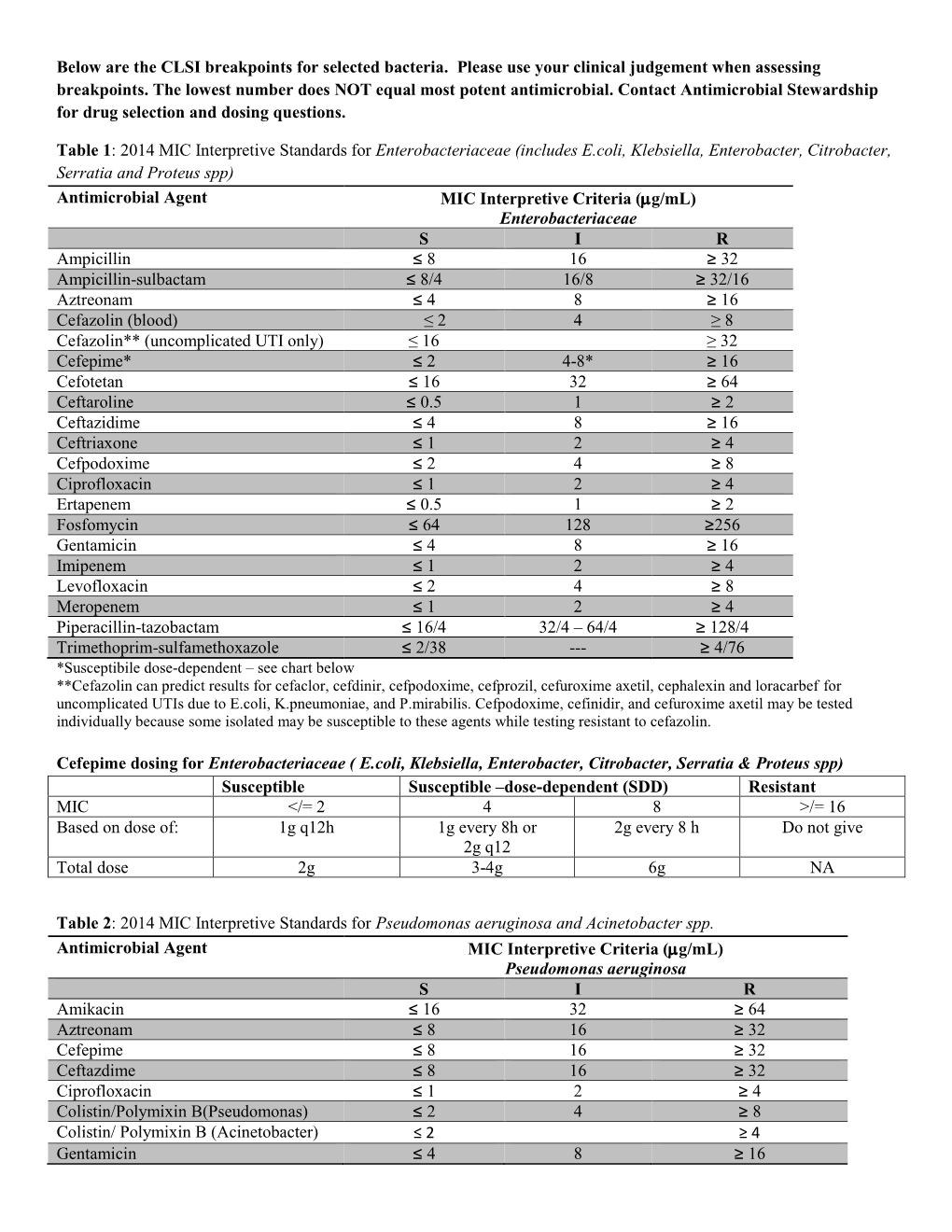
Clsi Breakpoints 2024 E Coli Suzy Leland, Laboratories should discuss immediate implementation of the clsi enterobacterales tzp breakpoint with their antibiotic stewardship program (asp), as the update provides a.
 Source: carylbmarlane.pages.dev
Source: carylbmarlane.pages.dev
Clsi Breakpoints 2024 E Coli Suzy Leland, Breakpoints are part of a system for categorising microorganisms as susceptible (s and i) and resistant (r) to agents approved for use in the treatment of infectious diseases.
 Source: www.researchgate.net
Source: www.researchgate.net
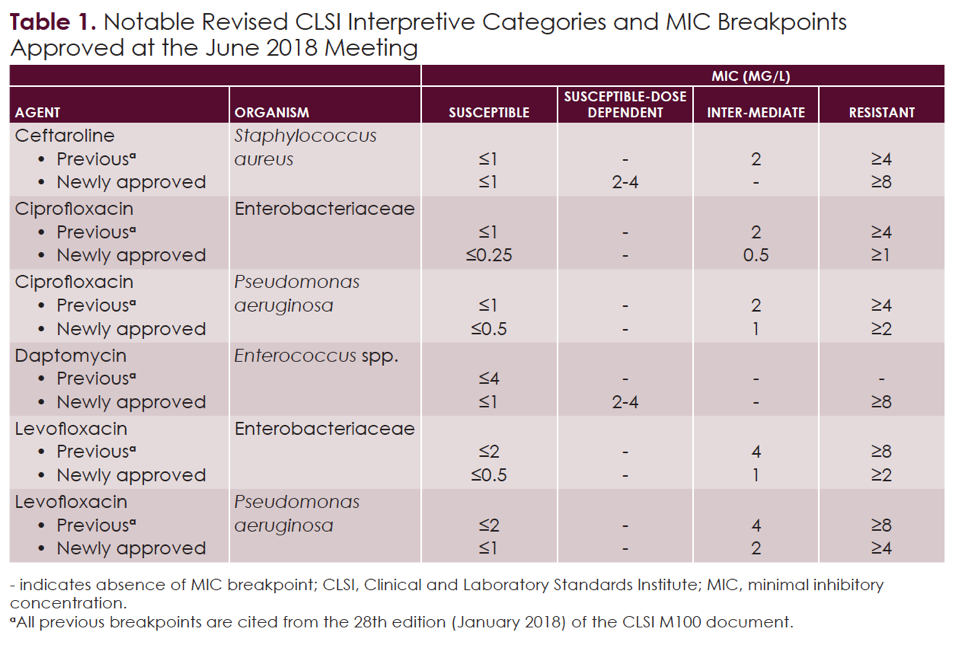
CLSi and eUCASt MiC breakpoints of antimicrobial agents assayed, This review outlines the process of setting and revising clinical and laboratory standards institute (clsi) breakpoints and summarizes breakpoints approved in.
 Source: clsi.org
Source: clsi.org
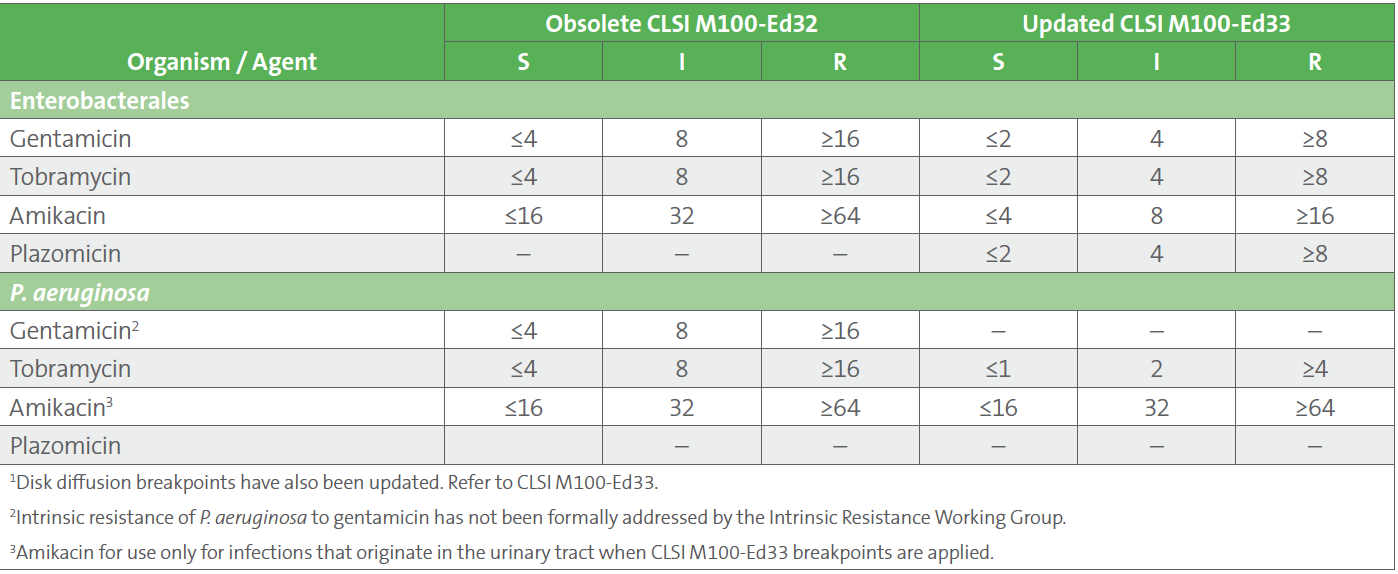
AST News Update June 2023 New! CLSI M100Ed33 Updated Aminoglycoside, Breakpoints are part of a system for categorising microorganisms as susceptible (s and i) and resistant (r) to agents approved for use in the treatment of infectious diseases.
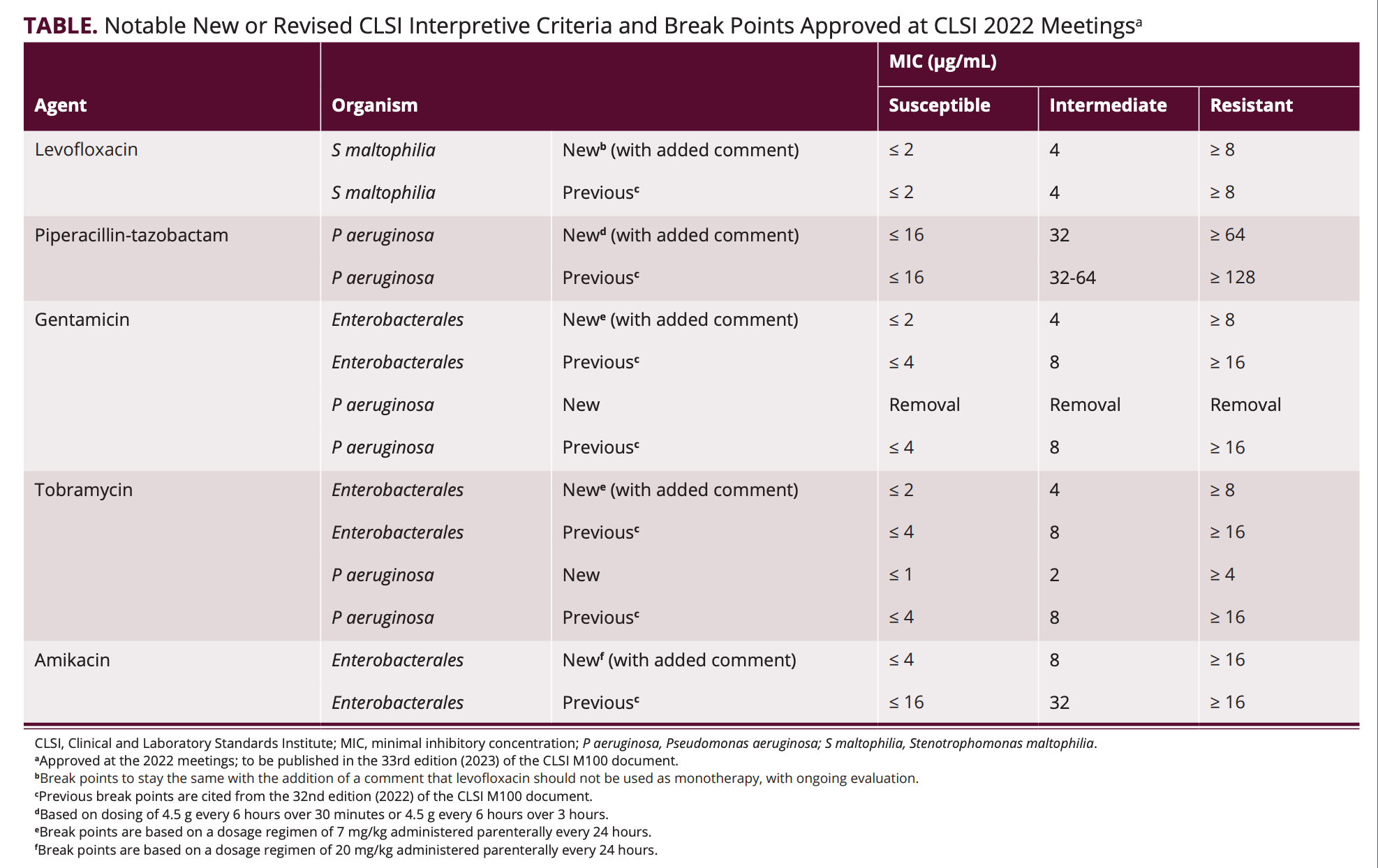 Source: www.contagionlive.com
Source: www.contagionlive.com
What’s New in 2022 From the CLSI on Antimicrobial, Effective january 2024, clinical laboratories performing antimicrobial susceptibility testing (ast) will be required to use breakpoints currently recognized by clinical and laboratory standards institute (clsi) or us food and drug administration (fda).
Clsi Breakpoints 2024 E Coli Exam Uta Libbey, Summarize highlights from clsi m100—performance standards for antimicrobial susceptibility testing, 34th edition standards for antimicrobial susceptibility testing (ast) and reporting.
 Source: www.scirp.org
Source: www.scirp.org
Antimicrobial susceptibility of strains of Enterobacteriaceae isolated, Some of the major updates discussed during the webinar.
Characteristics of strains used in this studya Download Scientific, New versions of the european committee on antimicrobial susceptibility testing (eucast) and the clinical and laboratory standards institute (clsi) breakpoint tables have been released for 2024.
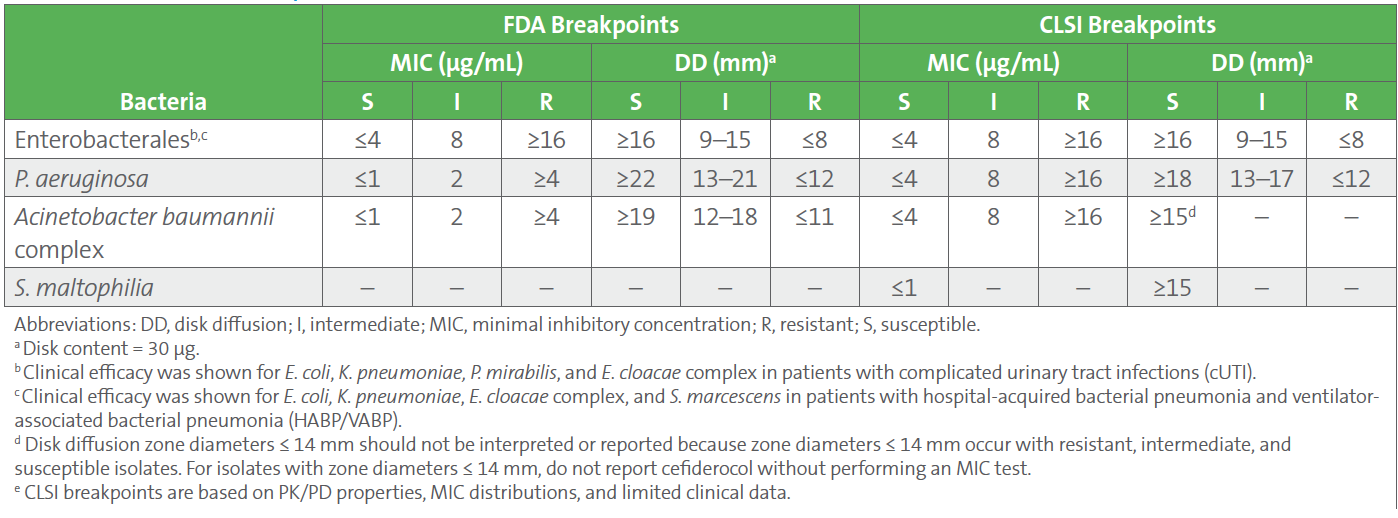 Source: clsi.org
Source: clsi.org
AST News Update June 2023 The Latest on Testing Cefiderocol, Effective january 2024, clinical laboratories performing antimicrobial susceptibility testing (ast) will be required to use breakpoints currently recognized by clinical and laboratory standards.
Posted in 2024